Thermodynamics
Molecular Motion Model
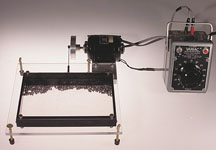
Note: The device should be angled so that when at rest the balls will settle along the metal plate. Also, it should be leveled so the "molecules" are equally distributed along the plate.
Boiling Water in a Paper Cup

Note: paper is ignited at 233°C
Heat Conductivity
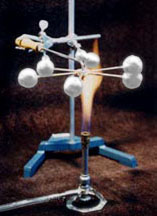
List of parts:
- Metal rod apparatus
- Wax
- Six 1/4-1/2" steel balls
- Bunsen burner
- Sparker
Thermal Expansion


Expanding Ball and Ring: At room temperature, the ball fits through the ring. When heated, the ball will no longer pass through.
Bimetalic Strip: made by welding together thin strips of brass and steel. These metals have different coefficients of thermal expansion. The strip curls when heated and re-straightens when cooled.
List of parts:
- Ball and ring
- Bimetalic strip
- Bunsen burner
- Spark lighter
- Beaker of water
- Liquid nitrogen (optional)
Convection Box

When a lit candle is placed under one clear chimney and a source of smoke (incense or punk) is placed above the other clear chimney, convection currents can be seen.
Note: The box demensions are 5"x8"x5". Use a camera for larger classes.
Beam of Cold
Two parabolic
mirrors are placed a couple of meters apart. The instructor puts a
thermometer in the focus of one of the mirrors, and a heat source (a
light bulb) in the focus of the other, and observe that the thermometer
reads an increase in temperature.
This result is trivial and expected by students. Now replace the heat
source with a cold source (copper ball filled with liquid nitrogen) and
notice the thermometer shows a decrease in temperature.
Instructor can ask students if we are dealing with a "beam of cold",
and discuss the second law of thermodynamics. This demonstartion can
explain some phenomena (for example, morning frost, etc.)

Note: Mirrors should be precisely aligned. For alignment look through the stainless steel tube and make sure you see the focus of the thermometer in the focus of the distant mirror. For reading the temperature, use the big screen LCD thermometer.

Note: Mirrors should be precisely aligned. For alignment look through the stainless steel tube and make sure you see the focus of the thermometer in the focus of the distant mirror. For reading the temperature, use the big screen LCD thermometer.
Radiometer

List of parts:
- Radiometer
- Light source with power supply
- The vanes spin because the black surfaces are hotter than the white ones, so they give off more energy to air molecules. It has nothing to do with radiation pressure.
- Use the Variac to vary the light intensity.
Triple Point of Water

This demonstration requires at least 20 minutes of lecture time. The setup is shown in the picture below.
Procedure:- A Petri dish half-filled with water is placed inside of the vacuum jar on a piece of thermally insulating material (styrofoam). Water evaporates very fast during evacuation, which can be harmful for the vacuum pump. To keep the pump safe, a water vapor trap is installed between the jar and the pump (see picture). This trap is a three-neck flask filled with blue Drierite crystals (Anhydrous Calcium Sulfate). When the Drierite crystals change color to pink, they do not absorb water any more and must be replaced.
- When the pump is turned on the water should start boiling at about 28mm Hg. Then in 5-10 minutes ice will form on the surface but water will continue to boil below it.
- Large bell jar
- Aluminum base and rubber pad
- Petri dish
- Three-neck flask filled with drying agent
- Vacuum pump and hoses
Constant Volume Gas Thermometer
This demonstration requires time and effort to setup, but is easy to
perform in an auditorium with the help of a student. A constant volume
of air is heated continuously and a large water manometer measures its
pressure. The instructor can obtain a roughly accurate plot of the
isochor process [ pt = p0( 1+1/273.15 T ) ] and extrapoloate it to absolute zero.

|
List of parts:
|

|
Steam Gun

Hero's Engine

Procedure:
- Fill flask to about 1/2 inch below nozzles. Seal the neck with a cork.
- Mount flask on the rod using test tubes as bearings as shown.
- Boil the water with the Bunsen burner. When enough steam is produced, the flask will spin. Don't let it run out of water.
- Hero's engine apparatus
- Base and rod
- 2 three finger clamps
- 2 short test tubes
- Bunsen burner and sparker
Stirling Cycle Hot-air Engine
